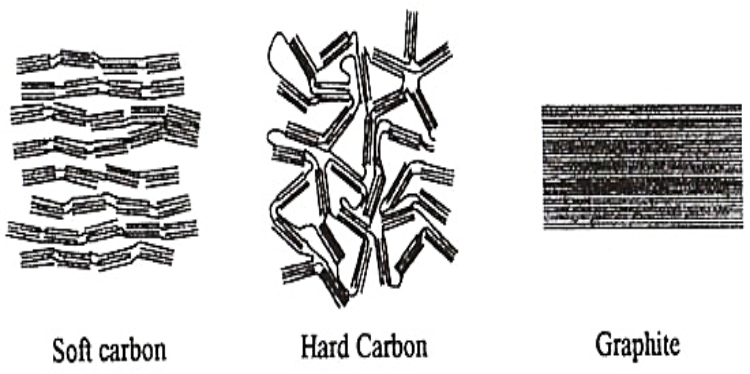
Hard carbon is a solid form of carbon that cannot be converted to graphite by heat treatment, even at temperatures as high as 3000 °C. It is also known as char or non-graphitizing carbon. More colloquially it can be described as charcoal. Hard carbon is produced by heating lignin and cellulosic biomass-based carbonaceous precursors to approximately 1000 °C in the absence of oxygen. Other precursors, such as polyvinyl chloride (PVC) and petroleum coke, produce soft carbon, or graphitizing carbon. Soft carbon can be readily converted to graphite by heating to 3000 °C. The physical properties of the two classes of carbons are quite different. Hard carbon is a low-density material, with extremely high microporosity, while soft carbon has little microporous. Hard carbon is extensively used as anode materials in electrochemical energy storage devices, such as supercapacitors, lithium-sulphur and Sodium-ion batteries that have been proven to be the most effective energy conversion and storage technologies for practical application. However, further development of these energy storage devices is hindered by their poor electrode performance. Carbon materials used in supercapacitors and batteries are often derived from non-renewable resources under harsh environments. Naturally, abundant biomass is a green, alternative carbon source with many desired properties.
About NBG
